DM168 Covid-19
Experts argue against ivermectin use
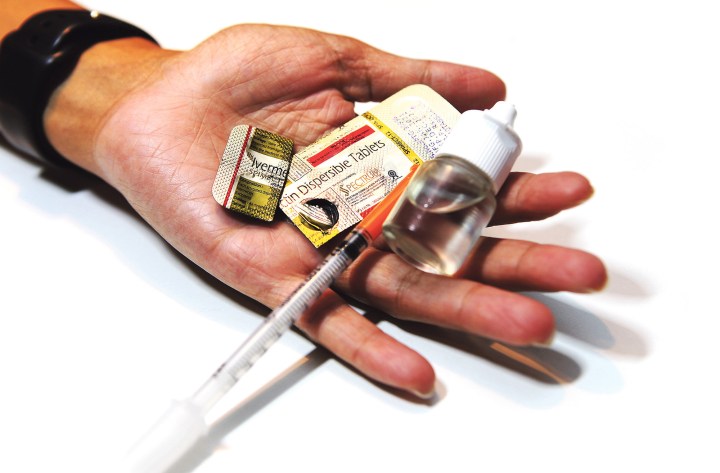
Although a recent court order has provided broader access to ivermectin for the treatment of Covid-19, many medical experts warn that there are still too many questions about the drug’s safety and efficacy.
First published in the Daily Maverick 168 weekly newspaper.
As the South African Health Products Regulatory Authority (Sahpra) settled several court applications on Tuesday 6 April to provide wider access to ivermectin for the treatment of Covid-19, the country’s infectious disease specialists insisted that this was not the right way to do it.
Despite battling through two brutal waves of the Covid-19 pandemic, South Africa’s frontline doctors, all infectious disease specialists, were among a group of esteemed medical experts who told the court that it was not in the public interest to allow broad access to ivermectin through legal action, insisting that evidence of the drug’s efficacy and safety were still lacking.
Dr Halima Dawood, the chairperson of the Infectious Diseases Society of Southern Africa (IDSSA), who was part of the team that treated the first Covid-19 case in the country, remained resolute that wide access for the use of a drug not scientifically proven to be effective against Covid-19 was not in the public interest.
In an affidavit before the Gauteng High Court filed by IDSSA with backup from the Treatment Action Campaign (TAC), the Southern African HIV Clinicians Society, the Allergy Society of South African, the South African Thoracic Society and health activist and current chairperson of the Covid-19 scientific committee, Dr Aslam Dasoo, Dawood stated:
“As yet, there has been no application to Sahpra for the registration of ivermectin for the prevention and treatment of Covid-19. In fact, ivermectin’s manufacturer has made [it] clear that it does not intend to make such an application.
“It has also expressed concern on the sufficiency of the evidence regarding its safety and efficacy in the treatment of human beings with Covid-19 symptoms. Without the required evidence, and without an application, Sahpra cannot register ivermectin to prevent or treat Covid-19.
“For a court to order the registration and/or use of ivermectin would be to undermine the medicines regulator and the process of medicines regulation in [SA]. We recognise that in times of a public health crisis, emergency measures can be taken.
“However, bypassing the regulator is neither in the short- nor … long-term interests of the country.”
And while the settlement order between the parties restricts access to those who have a prescription from a doctor, Dawood said that this was not the solution either.
“It cannot be left entirely to treating clinicians to decide whether medicine is safe and efficacious. This puts clinicians and the public in an invidious position. The role of a clinician treating a patient is to decide if an intervention has benefit for the individual patient, given their particular circumstances.
“The basis for this is knowing the performance of that intervention in similar, but not identical, populations. That information is established through evidence-based medicine, including the systems to evaluate the quality of that evidence, like medicines regulators (who possess the expertise to do so, as guided by the factors set out in the Medicines Act).
“It would place an undue burden and responsibility on doctors to take on the role of [both] regulator and clinician. The regulator protects patients by ensuring standards for drug quality, and protects doctors by ensuring there is an acceptable evidence base for them to safely prescribe a drug for specific indications,” she added.
The High Court in Pretoria has ordered that, apart from access to the drug through Sahpra’s compassionate programme, small batches of custom-made medicine containing ivermectin can now be legally prescribed “off-label” for the treatment of specific patients with Covid-19.
Ivermectin is an anti-parasitic drug. It has been registered in SA for the treatment of a skin condition and is used elsewhere to treat river blindness and other diseases caused by parasites in humans.
Since the outbreak of the global Covid-19 pandemic, there have been small trials, some of which show that it is effective against Covid-19 and some that it is not.
Dawood is also the head of Infectious Diseases at Greys Hospital, Pietermaritzburg.
“The pandemic has affected me in both my professional and personal life. Professionally, as the head of Infectious Diseases at Greys Hospital, a tertiary hospital that is situated in Pietermaritzburg, I led the team that managed the first patient in South Africa who was diagnosed on 5 March 2020 with the novel coronavirus (Covid-19). Since then we have managed [more than] 500 patients with Covid-19 at the hospital and have provided support and technical advice to colleagues within the referral catchment area,” she said, adding that she too had lost family members and colleagues during the pandemic.
“[Although] there is an urgent need for more impactful treatment, this needs to be the result of evidence-based medicine that is robust and unbiased.
“In the time when any intervention may be misconstrued as impactful treatment we need to resist the urge to recommend non-evidence-based practice in desperate times.”
Dasoo, the convenor of the Progressive Health Forum and convenor of the Science Reference Group for Covid-19, also supported the application not to allow wider access to ivermectin.
There were four applications before the court. In Case 2820/21, Dr George Coetzee, some of his patients and AfriForum argued for better access, against Sahpra and other decision-makers, like the Minister of Health.
In Case 3792/21 the African Christian Democratic Party (ACDP) and others argued for similar relief.
The IDSSA, TAC and other doctors’ organisations asked to join Case 6391/21 as friends of the court. This case was brought by the “I Can Make a Difference” Doctors and Medical Practitioners Group against Sahpra and others.
The fourth case, Case 9086, was brought by a group of pharmacies.
The legal arguments differed, but all four cases sought for patients to get access to ivermectin without the drug being registered by Sahpra and without the drug yet having been proven as safe or efficacious for the prevention and treatment of Covid-19.
Dawood said in papers before court that the access sought to ivermectin was “drastic”.
“We have lived and served [our] constituencies through health emergencies before. [We] have fought for access to medicines to save lives during the relevant periods in time, when people were dying daily [owing] to lack of access to life-saving medications. However, these struggles were conducted for medicine that had been proven to be safe and efficacious according to the clear and important standards set by evidence-based medicine.
“In a public health emergency, urgent action must be taken to gain access to safe, quality and efficacious medicine. But it is not in the interests of the public or of public health to disregard medicine regulation requirements,” she said.
She said there simply was not enough good evidence yet.
“There also continues to be an absence of consensus on certain critical factors on the use of ivermectin in the prevention and treatment of Covid-19. These are: the dose of ivermectin to use, what form of ivermectin to use (intravenous, pill or nasal spray), how long to use it and in which patients to use it. With these important factors remaining undetermined, there remain inherent risks associated with the use of the drug.”
Anele Yawa from TAC said they supported access to ivermectin through the controlled Section 21 programme.
Dasoo argued in papers before court that the compassionate use programme developed by Sahpra for ivermectin access was not justified by the evidence before it and was irrational.
The ACDP’s legal team argued that the compassionate use programme was inefficient and unworkable.
Steve Swart from the ACDP said in a statement that the new court order gave doctors the freedom to prescribe ivermectin without having to apply to Sahpra to do so. Compounding pharmacists and doctors can compound ivermectin for human use.
“The court order should also end the questionable use of the veterinary ivermectin product to treat humans, as well as stopping the burgeoning black market sale of the medicine.
“This is a great victory for freedom for doctors and the people of South Africa who have been suffering under the scourge of the Covid-19 pandemic, as it allows much broader access to the medicine, ivermectin,” he added.
The civil rights organisation AfriForum also welcomed the settlement.
Sahpra’s Yuven Gounden said they will only answer questions after they have consulted with their legal team.
What the research says about using ivermectin to treat Covid-19
This is a summary of a presentation done by Dr Andrew Hill from the University of Liverpool to the American National Institutes of Health.
He has been researching ivermectin for the global health agency Unitaid and the World Health Organisation. He was joined in his presentation by the doctors from the Front Line Covid-19 Critical Care Alliance.
Research in favour of ivermectin
There have been 18 small trials that involved a collective 2,100 patients. They were fairly small and fairly short.
These trials have separately shown a decrease in viral load and an improvement in markers doctors use to measure inflammation, faster times to hospital discharge and faster clinical recovery, as well as a 75% reduction in mortality rates.
This outcome was improved by higher doses over longer periods (five days) and not through a once-off dose – the normal dose for ivermectin when used to treat parasites.
Concern remains over higher doses having side effects.
Similar early successes were noted for drugs like chloroquine and lopinavir.
Studies in animals have shown that ivermectin can cause birth defects, so it is contra-indicated for pregnant women or women of child-bearing age.
In 2021 the National Institutes of Health changed its advice on ivermectin to reflect that it is neither for nor against the use of ivermectin to treat Covid-19.
Research against ivermectin
Dr Halima Dawood explained the current available information that South African doctors have on the use of ivermectin for the treatment of Covid-19.
In the most recent review conducted by the National Essential Medicines List Therapeutic Guidelines Sub-Committee on Covid-19 (“the committee”), only two randomised control trials out of 171 publications could provide any analysable information on the use of ivermectin for prevention of Covid-19.
The vast majority of studies that were included in the committee’s final analysis had not undergone peer review and contained very small numbers of participants. The studies were very difficult to judge together or compare, as the dosing strategies and primary outcomes were different.
Many of the trials did not investigate ivermectin alone against a placebo, but rather tested a combination of ivermectin and another medicine, either of which could have had an effect on the virus. This makes it almost impossible to tell whether any effect that is seen is due to ivermectin, the companion medicine or a combination of the two.
There is also no consensus regarding certain factors in the use of ivermectin for preventing and treating Covid-19, such as what dose of ivermectin to use, whether it should be intravenous, in pill form or via a nasal spray, how long it should be used for, and which patients should receive it.
On 4 March 2021, an article was published in the Journal of the American Medical Association detailing the latest clinical trial to test whether ivermectin worked as a treatment for mild Covid-19.
The study’s results were that by day 21, 82% in the ivermectin group and 79% in the placebo group had resolved symptoms. The conclusion that was reached by the authors was summarised as follows:
“Among adults with mild Covid-19, a five-day course of ivermectin, compared with placebo, did not significantly improve the time to resolution of symptoms. The findings do not support the use of ivermectin for treatment of mild Covid-19, although larger trials may be needed to understand the effects of ivermectin on other clinically relevant outcomes.”
“While the evidence on the efficacy of ivermectin is shifting all the time, the current and emerging evidence does not support the use of ivermectin for the prevention or treatment of Covid-19,” said Dawood.
What the court order on ivermectin says
A judge was not asked to rule on any of the issues because the matter was settled outside court. The settlement was then made a court order by a judge.
On access to ivermectin, the court order confirms that finished pharmaceutical products containing ivermectin can be accessed through the SA Health Products Regulatory Authority (Sahpra) compassionate programme.
The court order further states that pharmacists or doctors can compound (create) small amounts of ivermectin-containing medicine for specific patients on condition that a doctor has prescribed it. This opens the door for ivermectin to be prescribed for Covid-19. This is an “off-label” use, because the drug is currently only registered in SA for the treatment of a skin condition.
Sahpra has been ordered to report on progress, access and new information to the court every three months. DM168
This story first appeared in our weekly Daily Maverick 168 newspaper which is available for free to Pick n Pay Smart Shoppers at these Pick n Pay stores.
"Information pertaining to Covid-19, vaccines, how to control the spread of the virus and potential treatments is ever-changing. Under the South African Disaster Management Act Regulation 11(5)(c) it is prohibited to publish information through any medium with the intention to deceive people on government measures to address COVID-19. We are therefore disabling the comment section on this article in order to protect both the commenting member and ourselves from potential liability. Should you have additional information that you think we should know, please email [email protected]"







 Become an Insider
Become an Insider