Spotlight
The slow motion race for a TB vaccine versus the sprint for a Covid-19 vaccine
In recent months, the world has seen unprecedented investment in new vaccines. Yet, while a Covid-19 vaccine proven to be safe and effective may be less than a year away, a new tuberculosis vaccine might only be ready to be rolled out in a decade, despite a massive head start over Covid-19. Amy Green takes a closer look at the race for a new vaccine for the world’s top infectious disease killer.
If the world had forgotten the importance of vaccines, Covid-19 has served as a stark reminder. Less than a year after the emergence of SARS-CoV-2 (the virus that causes Covid-19), over a hundred vaccine candidates are under development and several large clinical trials are already under way, including some in South Africa. It is plausible that a safe and effective Covid-19 vaccine will be available within the next year or two, although only time and properly conducted trials will tell.
By contrast to the rapid development of Covid-19 vaccines, the most promising new tuberculosis (TB) vaccine might only be ready in a decade. This is despite the vaccine in question already having been tested in a large phase 2 trial of 3 575 people. The contrast is particularly striking when considering the latest World Health Organisation (WHO) figures announced last week, showing TB killed 58,000 people in South Africa in 2019, and around 1.4 million worldwide. If not worse than Covid-19, these numbers are at least comparable.
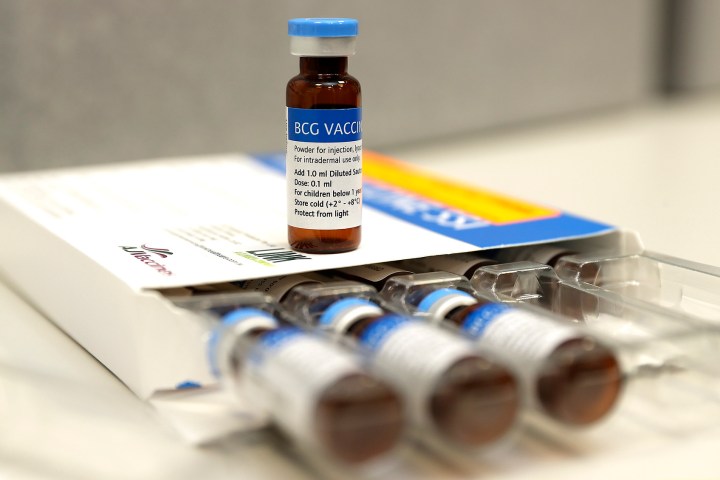
The first and only TB vaccine in existence is over a century old. Called BCG (Bacillus Calmette-Guerin), it is given to people at birth routinely in many countries around the world, including in South Africa, and is about 30% effective at preventing active TB disease, according to Professor Keertan Dheda, who heads up the University of Cape Town’s pulmonology department.
Since then, the race to find a new more effective TB vaccine has been anything but a race – more like a sloth’s attempt at one.
But the century-long effort has not been in vain. Today, there are 16 vaccine candidates in active clinical development, with most excitement about a vaccine candidate called M72.
Importance of vaccines
In its recent Global TB Report 2020, the WHO again stresses the importance of finding a new TB vaccine. Without it, it argues, TB rates will come down too slowly. According to the WHO, vaccines work “by training and preparing the body’s natural defences – the immune system – to recognise and fight off the viruses and bacteria they target. If the body is exposed to those disease-causing germs later, the body is immediately ready to destroy them, preventing illness”.
If the furore around a race towards a vaccine for Covid-19 doesn’t demonstrate the value of this prevention tool, consider the numerous recent measles outbreaks around the world caused by both a lack of access to, and a refusal to immunise children with the long-established measles, mumps and rubella vaccine.
Most notably, the Democratic Republic of Congo is home to the world’s largest measles outbreak in which, over the past two years, over 7 000 children have died. Here, low immunisation rates are to blame.
‘Governments not coughing up the money’
While the need for a safe and effective new TB vaccine is widely recognised, activists charge that governments have failed to invest sufficiently in the search for such a vaccine.
“For Covid-19 the United States [US] government alone signed contracts with a number of different vaccine makers worth over a billion dollars each. Take just one of those billion-dollar contracts; it exceeds the total amount that the entire field of TB vaccine development received in over 10 years,” says Mike Frick, TB co-director at the US advocacy organisation Treatment Action Group (TAG).
“Each year, funding for TB vaccines has been about 100 million dollars a year, and, overnight, the US government was able to award billion-dollar contracts to multiple vaccine developers for Covid-19. The money was always there – it’s a matter of priority-setting,” he says.
Speaking to Spotlight at the beginning of September, he says, “I think it’s telling that, at this point in the year, TB has killed more people than Covid-19 has. It’s not a contest of death. We don’t decide what to care about based on mortality numbers alone, but the comparison is still instructive because it shows that we should have been caring about TB all along.”
Dr Grania Brigden, Director of TB for The International Union Against Tuberculosis and Lung Disease (Union), makes a similar point. “The investment and political will to find a vaccine for Covid-19 is substantial, with the US alone investing $5.81 billion in vaccines,” she says. “The European Investment Bank will be investing 400 million euros and the European Union has pledged to invest at least 350-million euros in Covid vaccine development. Whereas the total investment for TB vaccine development in 2018 [from all sources] was just $109-million.”
Slow development of most promising vaccine candidate
According to Brigden, the world, in fact, does not care about TB, as evidenced by the slow development of the most promising vaccine candidate, M72.
“The M72 vaccine has had a very tortuous pathway to get it to where it is now. For example, the phase 2 trial was posted on ClinicalTrials.gov in December 2012, but the trial only started enrolling in August 2014,” she says.
In clinical trials, a phase 1 trial is a study conducted in a small number of people, up to a few dozen, typically to establish safety and appropriate dosages. Phase 2 trials are conducted in larger groups of people in order to gather more safety information and preliminary information on whether the treatment or vaccine is effective. While phase 2 treatment trials are usually quite small (often only a hundred or so participants), prevention or vaccine trials are typically much larger, since, as in the case of M72, most people in the trial will not develop TB with or without the vaccine and you thus have to include many more people in the trial in order to see a prevention effect.
M72’s preliminary phase 2 results made a splash when they were announced ahead of the United Nations High Level Meeting on Tuberculosis in September 2018, showing “the incidence of TB disease among HIV-negative, TB-infected adults was significantly lower among participants who received M72/AS01E compared with those who received placebo after two years of follow up”.
The full results were announced in October 2019 at the 50th Union World Conference on Lung Health in India and was published in the New England Journal of Medicine. It showed that three years after vaccination there were around 50% fewer cases of TB among study participants who received the vaccine, as opposed to a placebo.
If phase 2 trials establish efficacy, and the side-effects are not found to be too severe, the next step is to conduct a phase 3 trial to confirm the findings in an even larger group of people. It is typically only after positive findings from phase 3 trials that treatments or vaccines are approved to be marketed and used in healthcare systems.
Yet, says Brigden, it is now a year since M72 proved itself fit for phase 3 trials, and, despite “these promising results, the design of – and securing funding for – the phase 3 trial is still under way”. This is despite the fact that the non-profit, Bill & Melinda Gates Medical Research Institute, took over its development after it received M72’s licence from pharmaceutical company GSK in January this year. She says it is telling that taking the M72 vaccine candidate from phase 2 trials to planning of phase 3 trial – which are still not underway – has taken eight years.
It is, however, “commendable”, according to TAG’s Frick, that, while the phase 3 trial remains in the planning stages, another smaller study to see whether M72 is safe in people with HIV, is underway. “The goal is to generate enough safety and immunogenicity data so that people living with HIV can be included in the phase 3 study,” he says. This is important because, notes the WHO, people living with HIV have a much higher risk of developing TB. Their risk is between 16 and 27 times greater than those without HIV.
‘Austerity was false’
But why is it so difficult to secure funding for a “promising” vaccine candidate for the world’s leading infectious disease killer?
“The lack of investment in TB vaccine development is a result of chronic blunting to the horrors of a global epidemic that has been unfolding for decades among the socio-economically disadvantaged, particularly in developing countries with few resources at their disposal,” explains University of Cape Town Professor Mark Hatherill, Director of the South African Tuberculosis Vaccine Initiative. “By contrast, the global Covid-19 epidemic unfolded in a matter of weeks on the doorstep of wealthy industrialised countries with massive resources to develop vaccines against this sudden threat.”
According to Frick, what Covid-19 “illustrated is that, for too long we have accepted a false sense of fiscal austerity, that there was never enough money for TB vaccines”. “We were told to advocate and make an investment case, but there was always a reason not to increase funding. What Covid has clearly shown is that that austerity was false.”
Scientific reasons for slower development
While a lack of investment is clearly one reason for the slow pace of TB vaccine development, there are also good scientific reasons for TB research taking longer. This has to do with gaps in our understanding of TB, as well as the fact that TB is simply a disease that progresses much more slowly than for example Covid-19 or influenza.
We do not know why only 10 – 15% of humans who are infected with the TB bacterium “progress” to active TB disease, where they experience symptoms, says Hatherill. We also do not know why the remaining 85 – 90% “of infected people seem to enjoy lifelong protection”.
“TB vaccine trials are long slow projects, because TB is a chronic disease – a person may only develop TB disease years after first being infected. By contrast, a person may develop Covid-19 within days of being infected, so these trials can be conducted quickly during an epidemic,” explains Hatherill.
“TB is a complex organism and the fact that it has been around for thousands, if not millions of years, shows how difficult it has been for mankind to deal with,” adds Brigden. “Unlike Covid-19, TB is a bacterium, not a virus. TB has the ability to infect someone without making them sick or without the infected person being infectious to those around them. This means, for vaccine development, there are a number of different states that a vaccine could work on.”
For example, a vaccine could be aimed at people who are not infected at all to prevent any infection, it could be aimed at those who are infected to clear the infection or it could be aimed at preventing the progression from latent infection to active TB disease. She adds that there could even be a vaccine aimed at making TB treatment shorter and more effective.
“There remains a lot about TB that we do not fully understand. What exactly makes people go from having TB infection to TB disease? Is this a linear state or can people oscillate between TB infection and TB disease? The tools we have to detect TB infection are not ideal and cannot predict who with TB infection will go on to develop TB disease. These unknowns make vaccine development even harder,” she says.
Reviving BCG
According to Dheda, scientific work underway investigating the only TB vaccine in existence, the BCG vaccine, is particularly important. How that vaccine works is still not well understood, even though it is over 100 years old.
One key question being explored is how BCG efficacy can be improved. “While it is only 30% effective in preventing active TB, it has other advantages including that it reduces non-TB related childhood mortality by between two and four-fold,” says Dheda. “It is important to realise that even a small improvement in vaccine efficacy, say from 30 to 60%, will have a major impact on the global burden of TB.”
Studies investigating whether revaccinating people with BCG as adolescents or adults offer additional protection from becoming infected with TB are showing promise.
“A trial of BCG revaccination is testing the idea that adolescents who have not yet been infected with the TB bacterium may be prevented from becoming infected, as a means to reduce the number of people at risk of progressing from so-called ‘latent’ infection to TB disease,” says Hatherill.
A South African study, published in the New England Journal of Medicine in 2018 and conducted in a sample of almost 1,000 South African adolescents, found that BCG revaccination reduced TB infection rates by about 45%. A bigger trial is currently underway to confirm these findings.
Interestingly, UCT researchers are also investigating whether BCG efficacy can be improved if it is given via a different route, for example, inhaled through the lungs rather than being given as an injection under the skin which is the current route of administration.
“Several animal studies, including in monkeys, have shown that if BCG is given via the lung route (the same route of infection that TB uses), immunity is much more robust. Thus, if BCG is given via a simple dry powder inhaler, it could be a lot more effective than the current route of administration. Our group at UCT has shown, for the first time, that giving BCG via this route in those with different TB susceptibility profiles is safe and generates robust immune responses in both the lung and the blood,” says Dheda. The study was conducted among 106 South Africans and was published in the American Journal of Respiratory and Critical Care Medicine.
“We are now busy with a United Kingdom Medical Research Council-funded randomised trial that will test for the first time in humans whether giving BCG via the lung route is better than giving it via the skin route. We hope to have results from this study in about a year’s time,” says Dheda.
Repurposing BCG has major advantages including the fact that it is an old vaccine and is not on patent, making it relatively cheap. This also means patients won’t have to wait years for regulatory approvals to be finalised before being able to access the product, should the results of these BCG studies confirm that the new approaches work.
BCG supply issues
Although BCG is an old and well-established childhood vaccine, and is also used in larger volumes to treat bladder cancer, it has been plagued by global and local access issues, including stockouts.
According to Andy Gray from the University of KwaZulu-Natal’s pharmacy department, the stockouts have been due, in part, to the fact that there are only a handful of BCG manufacturers worldwide.
“Some of those firms have, over the past 10 years or so, had quality problems. Initially, for example, a Canadian plant discovered mould in their lines, and had to shut down,” he says.
After this incident, the owner of this plant, pharmaceutical giant Sanofi, decided to not reopen it and to exit the BCG market completely.
“In 2016, South Africa was unable to secure supplies from our usual registered supplier in Denmark, and had to resort to buying an unregistered product from India,” adds Gray.
According to a December 2019 Unicef report, following onsite inspections, the WHO “suspended, and subsequently delisted from vaccine prequalification, the product from another manufacturer (Green Signal Bio Pharma)”. The removal of this single India-based company “represents approximately a 30% reduction of global BCG vaccine supply availability”.
“Even the increased production of BCG by Merck of more than one hundred percent is still not enough to solve the enormous scarcity problem,” note the authors of a 2020 paper in the medical journal ImmunoTargets and Therapy.
BCG to prevent Covid-19?
BCG has been in the headlines in recent months, but not because of the advances in TB science or the chronic stockouts. Some hypothesise that BCG might also offer protection against Covid-19 infection, especially for high-risk groups like health workers and the elderly.
There are already 19 studies underway looking at BCG and Covid-19, according to Dr Arne von Delft, TB-survivor and co-investigator for a trial investigating whether BCG revaccination offers protection from Covid-19 in health workers.
While these studies are important, some experts have raised concerns about limited BCG stocks being steered away from those it has long been proven to help – children and people with bladder cancer.
Brigden makes the interesting point that greater investment in TB research in the past may have come in handy in our current fight against Covid-19.
“If we had invested in the basic science on TB and had funded the trials to investigate the non-specific immune effect of BCG then we would be able to better answer the question on whether BCG may have a protective benefit against Covid-19,” she explains.
“There are a lot of tools we have for TB that can help in the Covid response,” she continues. “For example, contact tracing [identifying at-risk individuals who have come into contact with infected persons], Gene Xpert [a diagnostic machine] and infection control [measures taken to stop the spread of infectious diseases, particularly in spaces like hospitals]. Investing in TB research and development [R&D] is investing in technologies for health in the broader sense, especially lung health and respiratory infections.”
Von Delft says we should not lose sight of the fact that TB remains the leading cause of death in South Africa, besides being the top infectious disease killer globally.
“Covid-19 is not competing for the top spot, it is working with TB to devastate even more lives, so we need shared solutions for these terrible pandemics,” he says. DM/MC
**NOTE: The Bill & Melinda Gates Medical Research Institute is mentioned in this Article. Spotlight receives funding from the Bill and Melinda Gates Foundation, a related organisation.
*This article was produced by Spotlight – health journalism in the public interest. Sign up for our newsletter.
"Information pertaining to Covid-19, vaccines, how to control the spread of the virus and potential treatments is ever-changing. Under the South African Disaster Management Act Regulation 11(5)(c) it is prohibited to publish information through any medium with the intention to deceive people on government measures to address COVID-19. We are therefore disabling the comment section on this article in order to protect both the commenting member and ourselves from potential liability. Should you have additional information that you think we should know, please email [email protected]"



 Become an Insider
Become an Insider
Great writing, as always
The limited supply issue of the BCG vaccine which could possibly be used for Covid immunity explains why everyone is skirting celebrating its possible success. All those countries that have had a BCG vaccination policy in place are the counties with the least number of infections and deaths….
Why the WHO does not promote more production of this simple vaccine is weird…pharma companies that already have the recipe should be churning these out instead of promising something new and untested in record time. Just my opinion.